The total number of electrons represented in a Lewis structure is equal to the sum of the numbers of valance electron on each individual atom Nonvalence electrons are not represented in Lewis structures so, Hydrogen is having 1 valance electronI quickly take you through how to draw the Lewis Structure of HF (Hydrogen Fluoride) I also go over the shape and bond angle · Lewis Dot Structure For Hydrogen Atom H Youtube Heres some of the guidelines for drawing dot structures Lewis dot diagram for hydrogen Lewis dot symbols and lewis structures Lewis dot structures during chemical bonding it is the valence electrons which move amongst different atoms Lets go ahead and look at another example

Reaktiva Syrearter Ros Superoxid Anjonhydroxid Radikal Vateperoxid Och Hypoklorit Anjon Lewis Electron Dot Stock Illustrationer Illustration Av Rotation Anticyclonic
Hydrogen peroxide lewis dot diagram
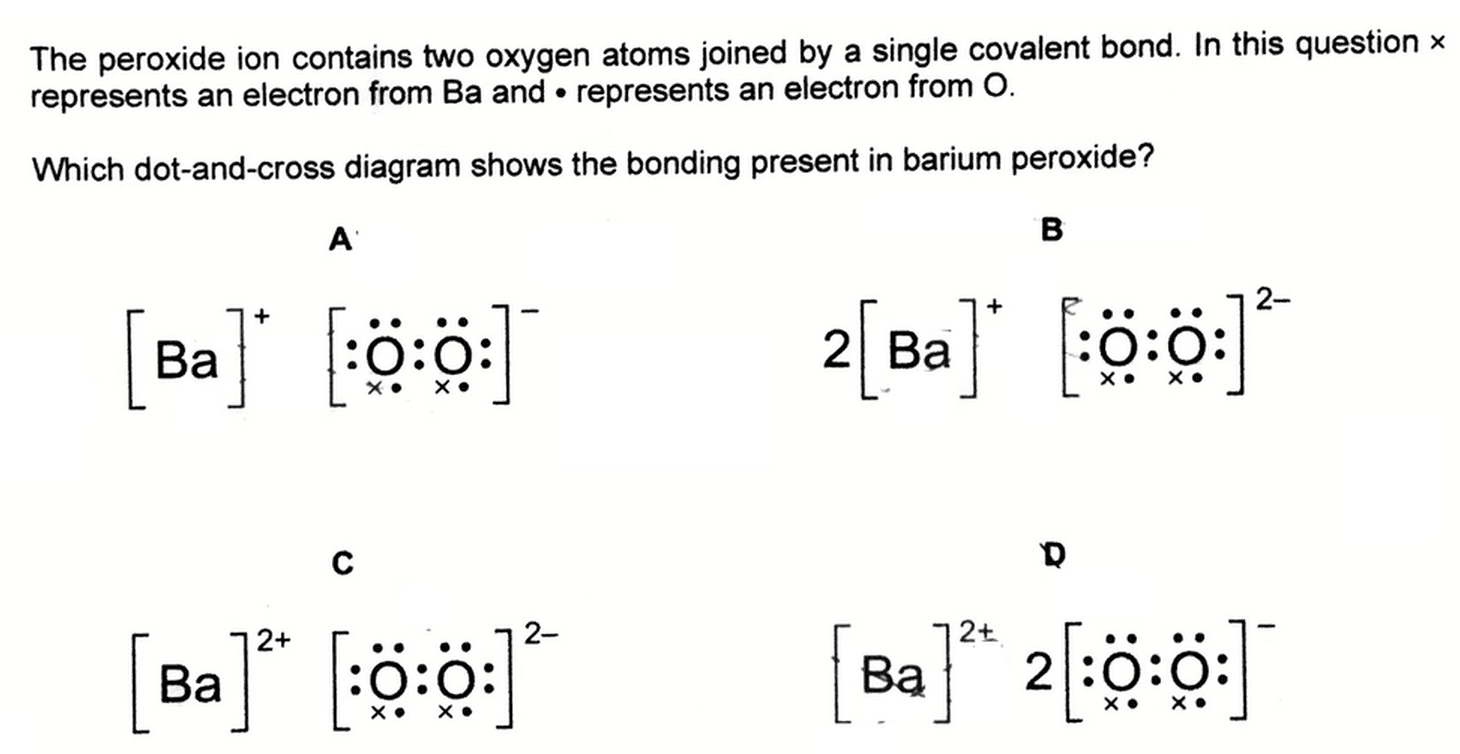
Hydrogen peroxide lewis dot diagram-Consider the faulting illustrated here in a normal fault, the block above the fault moves down relative to the block below the fault this fault motion is caused by tensional forces we might expect all but to occur at the sites of the faults a)volcanoes) b)earthquakes) c)mountain building) d)plateau formationQuestion B) Its Disproportionation Reaction Leads To The Formation Of Hydrogen Peroxide (H2O2) Start By Writing The Lewis Dot Structure Of H2O2, And Determine Its Shape I) Determine Point Group Symmetry For H2O2 Ii) Using Group Theory Approach, Determine The Irreducible Representations That Correspond To The Group Valence Shell Orbitals Of The Central O Atoms



Lewis Structures Simple Organic Compounds Janet Gray Coonce
Draw the Lewis structure for hydrogen peroxide, H 2 O 2 Learn this topic by watching Lewis Dot Structures Neutral Compounds Concept Videos All Chemistry Practice Problems Lewis Dot Structures Neutral Compounds Practice Problems · H2o2 lewis structure contains two o h bond and one o o bond connected with a single bond also 4 total lone pairs present in the lewis structure of h2 the hydrogen peroxide lewis diagram is very simple and the procedure for drawing it same as the other molecules let's see how to draw this step by stepExplain its role in bonding between atoms 2) Indicate how many electrons must be gained or lost by each of the following atoms to achieve a stable electron configuration, eg 3 lost, 2 gained, etc?
· Lewis Dot Structure for Hydrogen (H)Atom To get the valence electrons of hydrogen,we need to look at the electronic configuration of hydrogen H (1)=1s¹ The highest value of principal quantum number here is n=1Lewis Dot Structure Mega Worksheet 1) What is the octet rule?0806 · Lewis Electron Dot Structure Of Hydrogen Peroxide H2O2 Tx Academy Class 11 Chemical Bonding
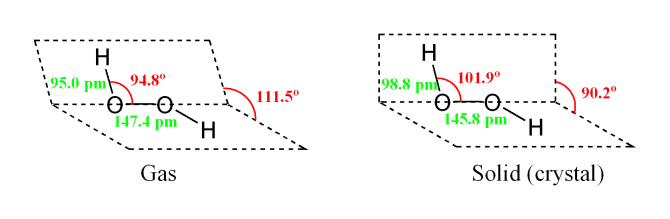
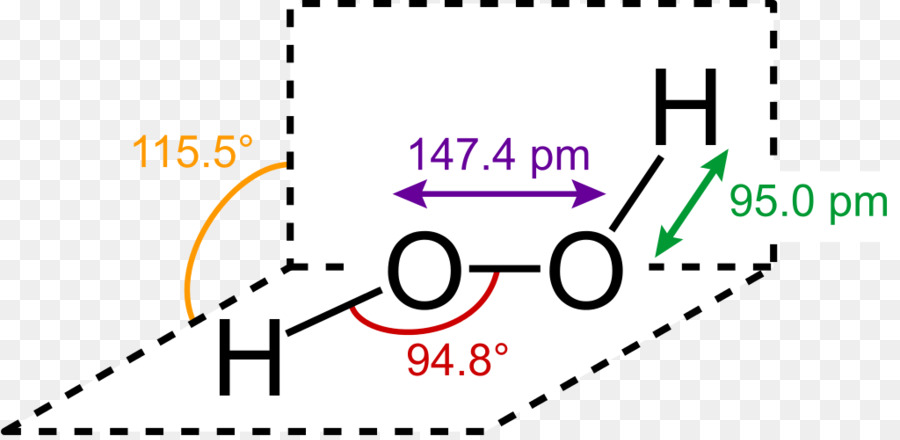
A video explanation of how to draw the Lewis Dot Structure for the Peroxide Ion, along with information about the compound including Formal Charges, Polarity · Hydrogen Peroxide consists of 2 hydrogens and 2 oxygen atoms arranged in an open book like structure with bent OHO bonds The electronegativity of oxygen is around 344 and that of hydrogen is 22 The difference between the electronegativity of O and H atoms causes the OH bond to be polarFully explain how you know from its structure Think carefully This is not trivial Just saying "symmetrical" or "not symmetrical" is insufficient


File H2o2 Structure Png Wikimedia Commons



Solved 19 A Student Proposed The Lewis Structure To The Chegg Com
Hydrogen peroxide dosedependently increased the intracellular ROS generation, which was significantly repressed by HW, both in the cytoplasm and nuclei LIVE/DEAD staining and our original cell viability dyeextraction assay showed that HW significantly protected HGF cells from hydrogen peroxideinduced cell deathHydrogen peroxide H2O2 CID 784 structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more · The chemical name for H2 O2 is hydrogen peroxide Its Lewis structure shows us where the valence electrons are located in the molecule, which can aid us in Count the number of electrons, add single bonds between the atoms, using two electrons per bond, arrange the remaining electrons around the


Barium Peroxide Dot Cross Diagram



Construct A Lewis Structure For Hydrogen Peroxide H2o2 In Which Each Atom Achieves An Octet Of Electrons
Create Sodium peroxide appears as a yellowwhite to yellow granular solid Mixtures with combustible material are readily ignited by friction, heat, or contact with moisture May vigorously decompose under prolonged exposure to heat, causing the rupture of the containers · Draw a Lewis dot electron dot structure for a molecule of hydrogen peroxide, H2O2The chemical name for H 2 O 2 is hydrogen peroxide Its Lewis structure shows us where the valence electrons are located in the molecule, which can



Electron Structures Of Common Reactive Oxygen Species Reactive Oxygen Species Study Notes Water Branding



Jolzfmcez9bcom
The Lewis structure of hydrogen peroxide is Books Physics NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless Chemistry NCERT P Bahadur IITJEE Previous Year Narendra Awasthi MS Chauhan Biology NCERT NCERT Exemplar NCERT Fingertips Errorless Vol1 Errorless Vol2 Maths NCERT RDDraw a Lewis electrondot structure for hydrogen peroxide, H 2 O 2, which is used to bleach hairMasteringChemistry with Pearson Etext Valuepack Access Card for Introductory Chemistry Atoms First (5th Edition) Edit edition Problem 34PP from Chapter 10 Draw a Lewis dot diagram for H2O2 (hydrogen peroxide), and u


7 E Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts



Hooh Lewis Structure How To Draw The Lewis Structure For Hydrogen Peroxide Youtube
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How works Test new features Press Copyright Contact us Creators · Let's do the Lewis structure for H2O2 Hydrogen Peroxide, also called dihydrogen dioxide On the periodic table, Hydrogen's in group 1 so it has 1 valence electron, but we have two of them, so we need to multiply by 2 What is the Lewis structure for H 2 O 2?Hydrogen peroxide is a chemical compound with the formula H 2 O 2In its pure form, it is a very pale blue liquid, slightly more viscous than waterIt is used as an oxidizer, bleaching agent, and antisepticConcentrated hydrogen peroxide, or "hightest peroxide", is a reactive oxygen species and has been used as a propellant in rocketryIts chemistry is dominated by the O–O bond


Stabilization Of Hydrogen Peroxide By Hydrogen Bonding In The Crystal Structure Of 2 Aminobenzimidazole Perhydrate Crystengcomm Rsc Publishing


Hydrogen Peroxide Molecule Chemical Compound Lewis Structure Png Clipart Catalysis Chemical Bond Chemical Compound Chemical Decomposition
C Hydrogen peroxide {eq}(H_2O_2) {/eq} d Review what a Lewis dot diagram is and discover how to draw a Lewis dot structural formula for compoundsLewis Dot Structures Lewis Dot Structure of Atoms Link Determining Shape Video Lewis Structure Hydrogen Fluoride HF Lewis Structure Nitrogen Oxide NO Lewis Structure Nitrosonium() Ion NO Lewis Structure Cyanide() Ion CNLewis Structure Peroxide(2) Ion O 2 2Lewis Structure Hydroxide() Ion OHLewis Structure DioxygenHydrogen peroxide, H2O2, the oxygen atoms are in the center (H–O–O–H) 7 In drawing Lewis structures for relatively small molecules and polyatomic ions, the structures tend to be more stable when they are compact and symmetrical rather than extended chains of atoms EXAMPLE Write the Lewis structure for CH2O where carbon is the central
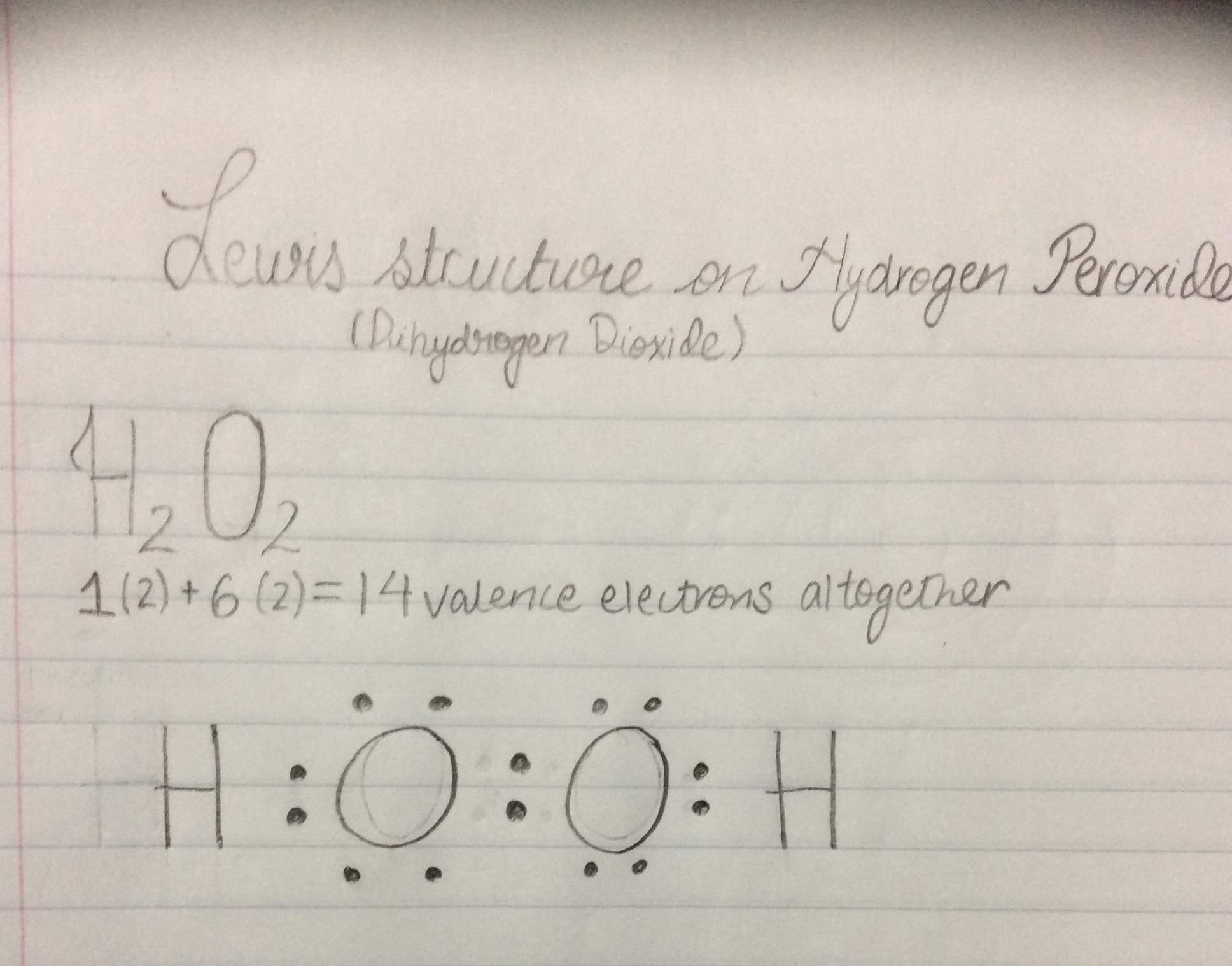


Hazards Of Hydrogen Peroxide In Hair Dye


Lewis Dot Structure Mega Worksheet
Lewis Dot Structures of Molecules Lab Name_ _____ Draw the Dot Structures for each of the following molecules (Module 4 Part B) Use alternating colored pencils or pens to Hydrogen Peroxide H 2 O 2 Water H 2 O also try (not all single bonds) O 2 H 2 N 2 2Draw the 3D Lewis electron dot structure for H2O2, including lone pairs Is hydrogen peroxide a polar or nonpolar molecule?Hydrogen Peroxide The chemical name for H 2 O 2 is hydrogen peroxide Its Lewis structure shows us where the valence electrons are located in the moleculeWhat is the Lewis structure


Compare The Structures Of H2o And H2o2 Class 11 Chemistry Cbse
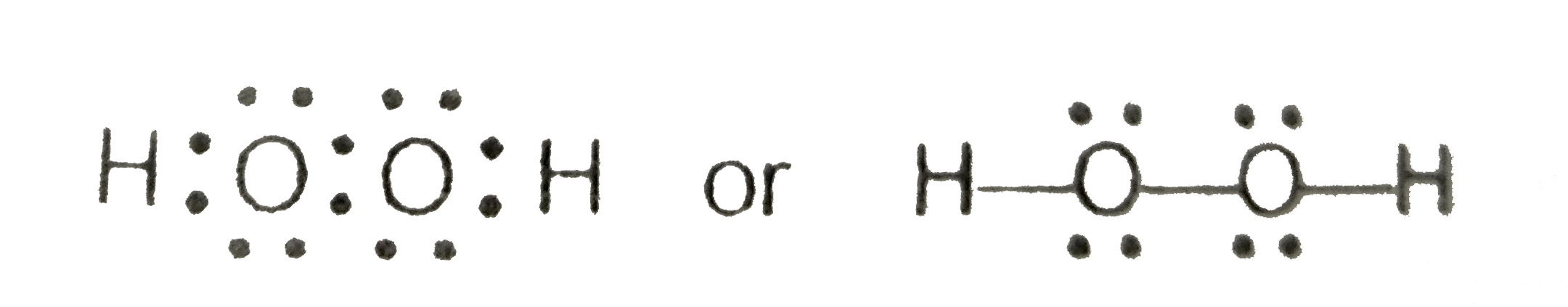


Write The Lewis Structure Of Hydrogen Peroxide
PubChem is the world's largest collection of freely accessible chemical information Search chemicals by name, molecular formula, structure, and other identifiers Find chemical and physical properties, biological activities, safety and toxicity information, patents,04 · The lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond What is the dot and cross diagram for hydrogen bromide Lewis electron dot diagrams for ions have less for cations or · A stepbystep explanation of how to draw the H2O2 Lewis Dot Structure (Hydrogen peroxide) Note that the H2O2 Lewis structure is frequently used on tests a


Chem4kids Com Hydrogen Orbitals And Compounds


Hydrogen Peroxide Features And Uses
· A stepbystep explanation of how to draw the HOOH Lewis Dot Structure For the HOOH Lewis structure, calculate the total number of valence electrons for the · Lewis Dot Diagram For H2 General Wiring Diagram This type of lewis dot structure is represented by an atomic symbol and a series of dots see the following examples for how to draw lewis dot structures for common atoms involved in covalent bonding example 1 draw the lewis dot structure for the hydrogen atom since hydrogen is in group i it has one (1) valenceWrite the Lewis structure of hydrogen peroxide Write the Lewis structure of hydrogen peroxide Books Physics NCERT DC Pandey Sunil Batra HC Verma Pradeep Errorless Chemistry NCERT P Bahadur IITJEE Previous Year Narendra Awasthi MS Chauhan Biology NCERT NCERT Exemplar NCERT Fingertips Errorless Vol1 Errorless Vol2


Hydrogen Peroxide H2o2 Chemspider



Hydrogen Peroxide Simple English Wikipedia The Free Encyclopedia
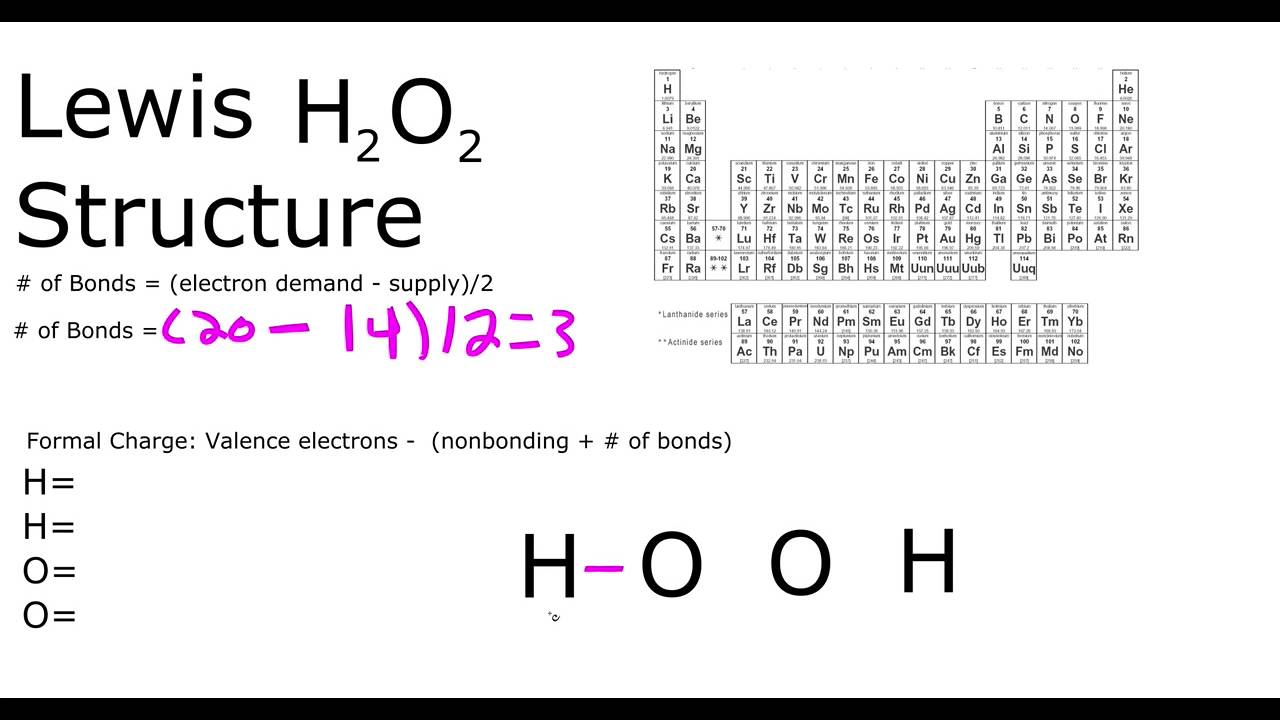
Every chemistry student has to learn how to draw Lewis Dot Structures The key is to understand the steps and practice Lewis Structures are important to learn because they help us predict the shape of a molecule how the molecule might react with other molecules the physical properties of the molecule (like boiling point, surface tension, etc)Hydrogen belongs to group IA It has one valence electron Oxygen belongs to VIA group It has six valence electrons Total number of valence electrons in is 2(1) 2(6)=14 Write the skeletal structure of the ions Put remaining electrons as lone pairs starting with oxygen That gives Lewis dot structures of the molecule as shown aboveHomework Help What's your question?
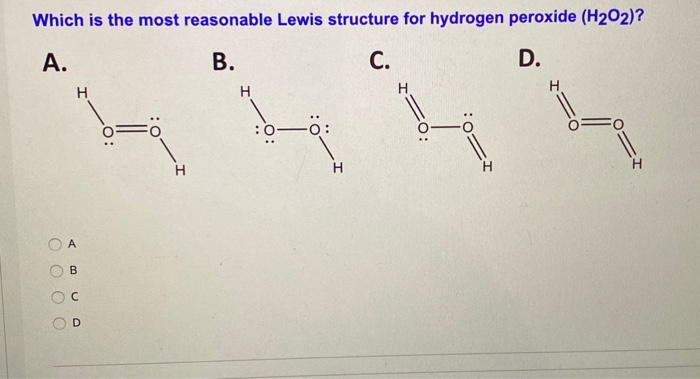


Solved Which Is The Most Reasonable Lewis Structure For H Chegg Com
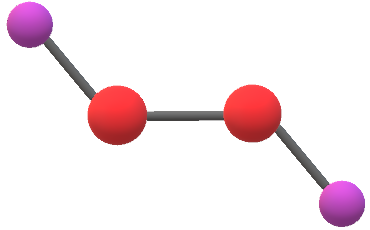


H2o2 Lewis Structure Hydrogen Peroxide Molecular Geometry Polarity
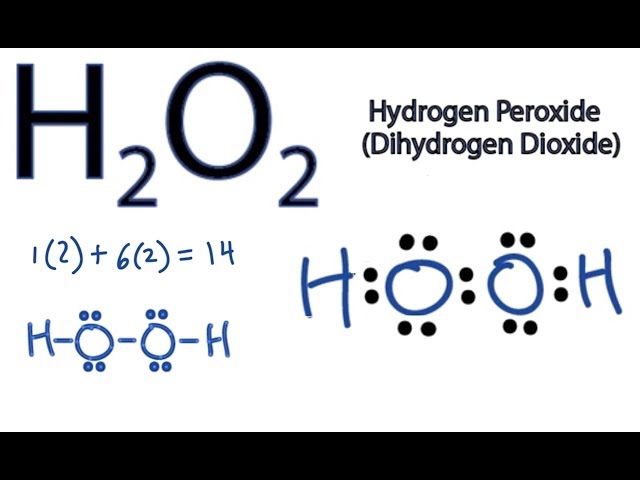
How to draw Hydrogen Peroxide (H2O2) lewis structure or Electron dot structure H2O2 lewis structure contains two OH bond and one OO bond connected with a single bond Also 4 total lone pairs present in the lewis structure of H2Draw A Lewis Dot Structure For Hydrogen, Lewis Dot Structure of HF (Hydrogen Fluoride) , NH3 Lewis Structure How to Draw the Dot Structure for, HSO4 Lewis Structure How to Draw the Lewis Structure for, CH4 Lewis Structure How to Draw the Dot Structure for · Get the detailed answer What is the correct lewis structure for hydrogen peroxide, H2O2?
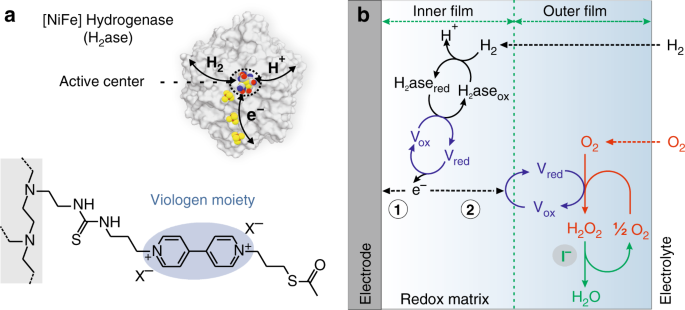


Suppressing Hydrogen Peroxide Generation To Achieve Oxygen Insensitivity Of A Nife Hydrogenase In Redox Active Films Nature Communications



Hydrogen Peroxide H2o2 Molecule Chemical Structure Hooh Is Royalty Free Cliparts Vectors And Stock Illustration Image
Lewis Electron Dot Structure Hydrogen, HI Lewis Structure How to Draw the Dot Structure for HI, CH4 Lewis Structure How to Draw the Dot Structure for, H2Se Lewis Structure How to Draw the Dot Structure for, Methanol Lewis Structure How to Draw the Lewis StructureThe hydrogen peroxide is activated by acetamide and disodium hydrogen phosphate or by an arsenic compound The overall reaction results in the formation of methyl ethyl ketazine in high yield The mechanism requires the activation of ammonia and hydrogen peroxide as these two reactants, unlike ammonia and hypochlorite in the Bayer process, do not react togetherPricing Log in Sign up Chemistry Dalia O'Kon 11 Dec 19 What is the correct lewis structure for hydrogen peroxide, H 2 O 2?


Lewis Structure Of H2o2


Hydrogen Peroxide Franzcalvo
This is the Lewisdot structure of hydrogen peroxide Here you have two oxygen atoms and the overall required electrons are 6*22=14,An atom of an element is three times as heavier as the mass of an atom of carbon12Hydrogen Peroxide (H 2 O 2) Lewis Structure Lewis structure of Hydrogen peroxide (H 2 O 2) contains two OH bonds and one OO bond Also, there are two lone pairs on each oxygen atom Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2Draw Lewis structure(s) showing all possible equivalent resonance forms for the hydrogen peroxide molecule ( H 2 O 2 ) Draw Lewis structure(s) showing all possible equivalent resonance forms for the hydrogen peroxide molecule ( H2O2 )Draw one structure per sketcher box, and separate any added sketcher boxes with the â†" symbol
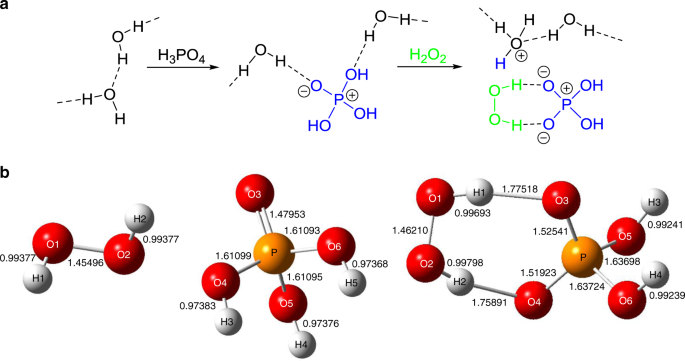


Photocatalytic Hydrogen Peroxide Splitting On Metal Free Powders Assisted By Phosphoric Acid As A Stabilizer Nature Communications



How Many Bonding Electrons Are Between The Clutch Prep
A) Sr b) Sb c) Si d) S e) Se f) Xe 3) Which of the following pairs of elements will not form ionic compounds? · Get the detailed answer What is the Lewis structure for the molecule hydrogen peroxide, H2O2?



Hydrazine Nh2 Nh2 Hydrogen Peroxide H Clutch Prep



Two Electron Oxidation Of Water To Form Hydrogen Peroxide Sensitized By Di Hydroxo Porphyrin Geiv Complex Under Visible Light Irradiation Sciencedirect



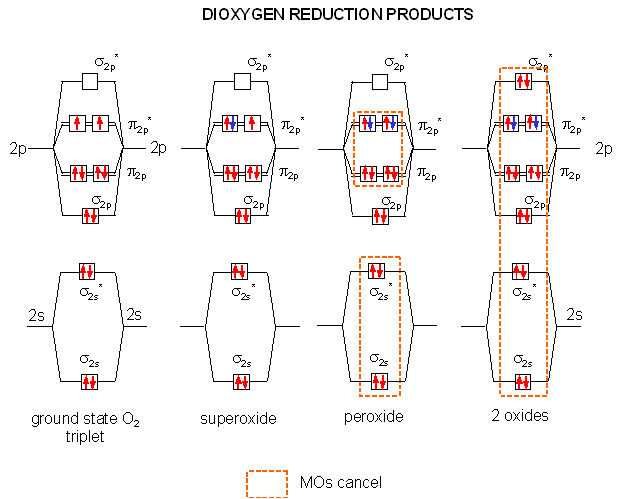
Reduction Of O2 To H2o And Its Free Radical Intermediates A Lewis Download Scientific Diagram



H2o2 Lewis Structure


Why Is Hydrogen Peroxide H2o2 Instead Of Ho Quora


Hydrogen Peroxide H2o2 Pubchem


Chapter 3



Hydrogen Peroxide Structure Properties Uses With Questions Videos
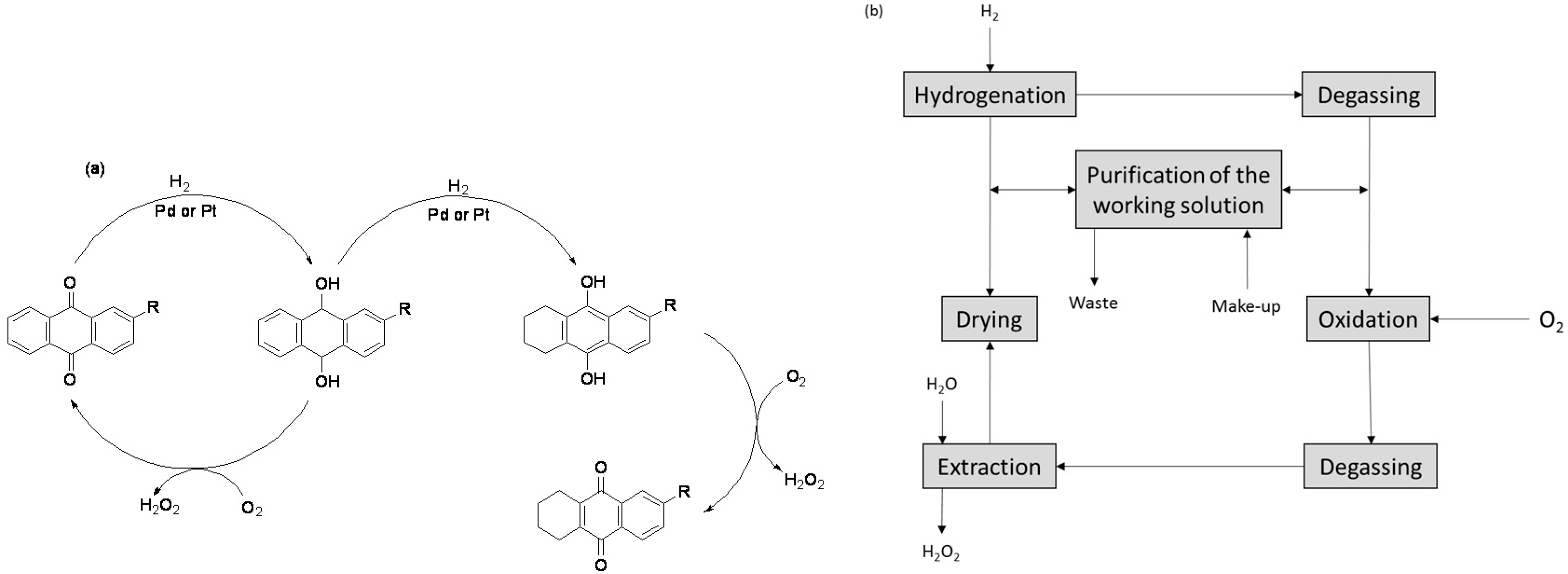


Catalysts Free Full Text Recent Advances In The Direct Synthesis Of Hydrogen Peroxide Using Chemical Catalysis A Review Html



Production Of Superoxide And Hydrogen Peroxide From Specific Mitochondrial Sites Under Different Bioenergetic Conditions Journal Of Biological Chemistry



Hydrogen Peroxide H2o2 Lewis Structure Novocom Top
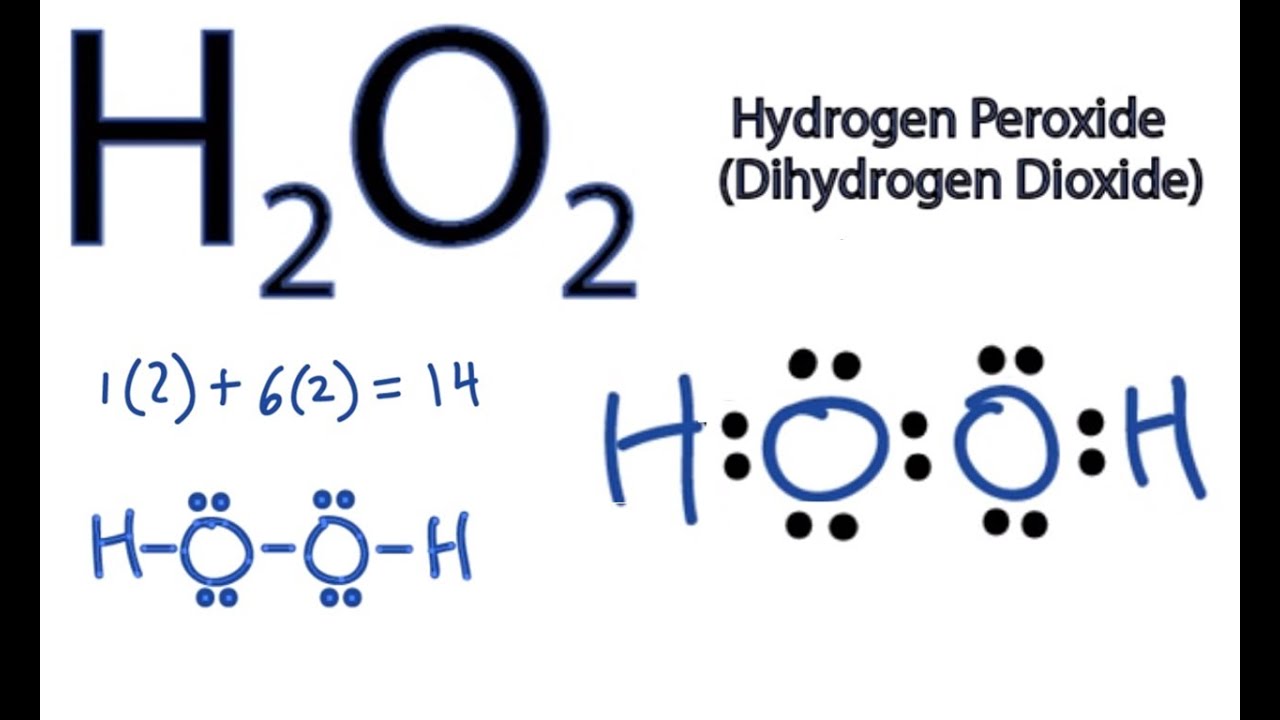


H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Chemical Bonding Success In Chemistry


Redox Reactions



Draw The Lewis Structure For Hydrogen Pero Clutch Prep
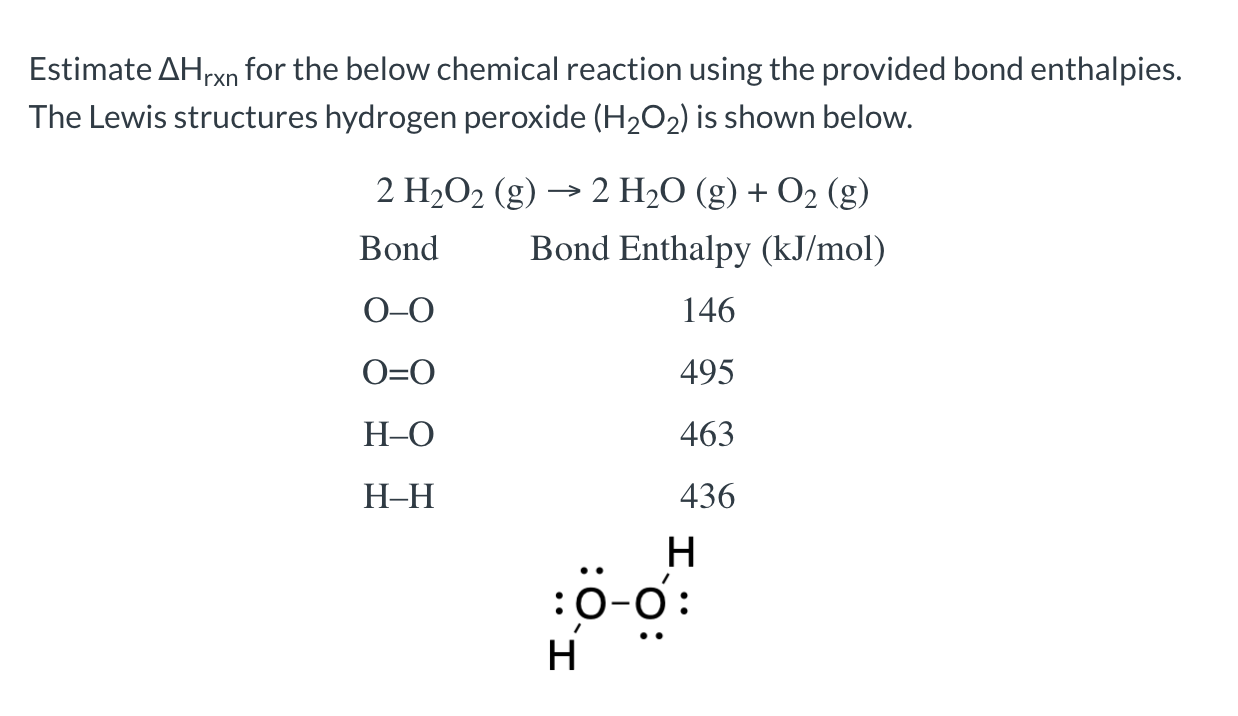


Solved Estimate Ahrxn For The Below Chemical Reaction Usi Chegg Com
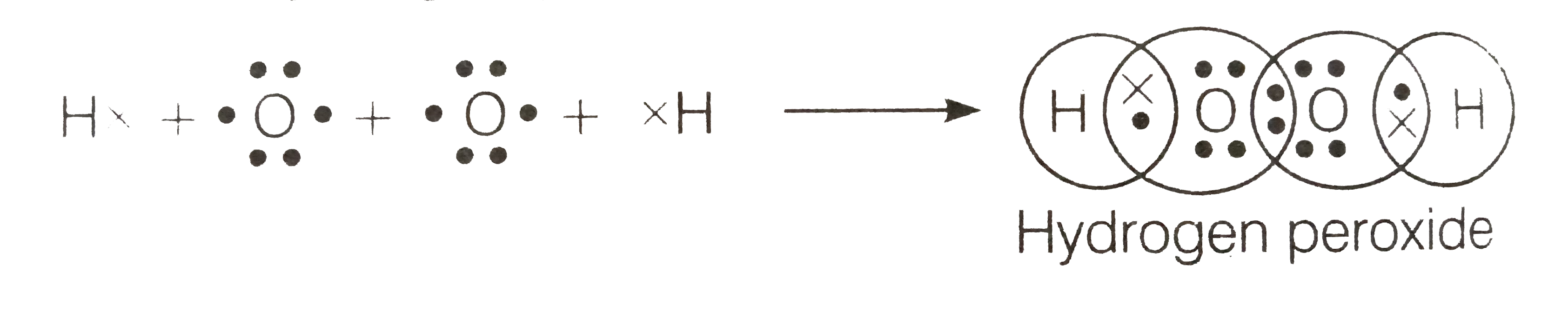


Write The Lewis Structure Of Hydrogen Peroxide


Peroxide Wikipedia
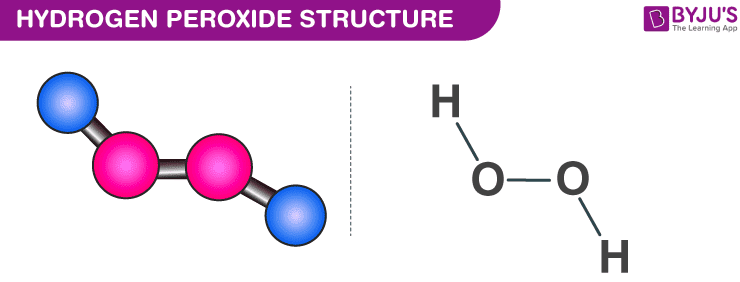


Hydrogen Peroxide H2o2 Structure Preparation Properties Uses
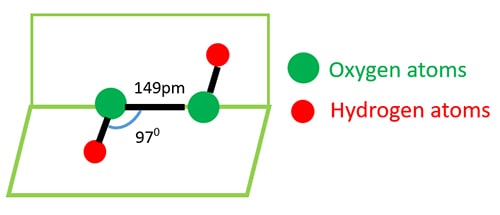


Hydrogen Peroxide Reactions And Physical Properties H2o2



What S Your Question Pricing Log Insign Up Chemistry 1 Answer 0 Watching 135 Views Titus Jacobson 28 Nov Draw The Lewis Electron Dot Structure For Hydrogen Peroxide Which Is Used To Bleach Hair Answer Watch 1 Answer 0 Watching 135 Views For


Hydrogen Peroxide Wikipedia
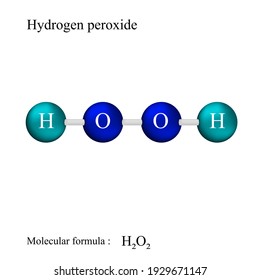


Lewis Structure High Res Stock Images Shutterstock



Hydrogen Peroxide Molecule And Its Lewis Dot Electron Dot Structure H2o2 Formal Science Chemistry Essay Essaysusa Com



The Lewis Structure For H2o2 Novocom Top
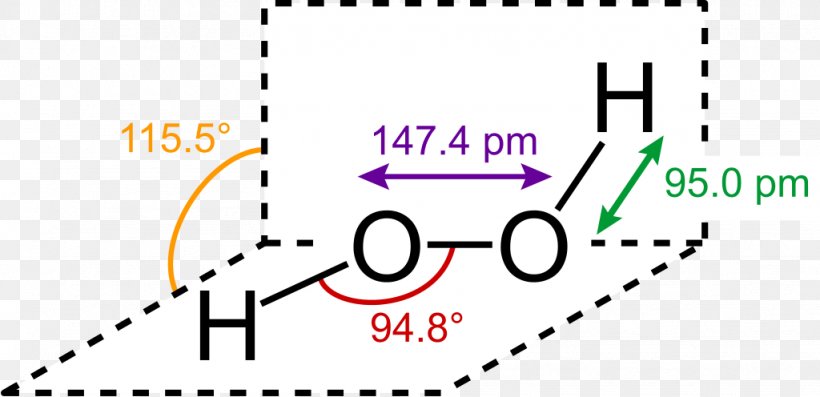


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png 1024x496px Lewis Structure Area Brand Chemical Bond Chemical


Chemistry Cartoon Png Download 1024 496 Free Transparent Lewis Structure Png Download Cleanpng Kisspng


H2o2 Lewis Structure Youtube



Write The Lewis Structure Of Hydrogen Peroxide Youtube



The Lewis Structure For H2o2 Novocom Top



The Diagram Shows The Structure Of Hydrogen Peroxide What Is The Total Number Of Electron Used For Brainly In



Lewis Structures Simple Organic Compounds Janet Gray Coonce



H2o2 Lewis Structure Shape Novocom Top



Open Source Physics Singapore Chemical Bonding Dot And Cross Diagrams Polyatomic Ions Dot And Cross Diagram



Lewis Dot Structure Of H2o2 Novocom Top



H2o2 Lewis Structure Hydrogen Peroxide Molecular Geometry Polarity


H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Youtube


Add Your Page Title



Introduction To Chemical Bonds Ck 12 Foundation



Draw A Lewis Dot Structure Of D2o2 Novocom Top



Lewis Electron Dot Structures Chemistry For Non Majors
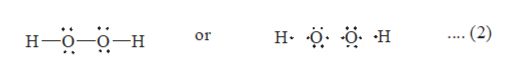


Answered Draw A Lewis Dot Electron Dot Structure Bartleby
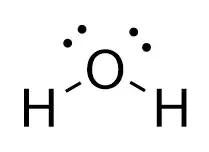


Why Does The Extra Oxygen Atom In Hydrogen Peroxide H2o2 Make It An Antiseptic While Water H2o Is Not Antiseptic Quora



Reaktiva Syrearter Ros Superoxid Anjonhydroxid Radikal Vateperoxid Och Hypoklorit Anjon Lewis Electron Dot Stock Illustrationer Illustration Av Rotation Anticyclonic



Hydrogen Peroxide


The Hydrogen Peroxide Guide By Sophia Petrou Infographic


Efficient Hydrogen Peroxide Synthesis By Metal Free Polyterthiophene Via Photoelectrocatalytic Dioxygen Reduction Energy Environmental Science Rsc Publishing



Whose Dog Is This Hydrogen Peroxide Disproportionation Reactions Oxidation Numbers Electronegativity More The Bumbling Biochemist
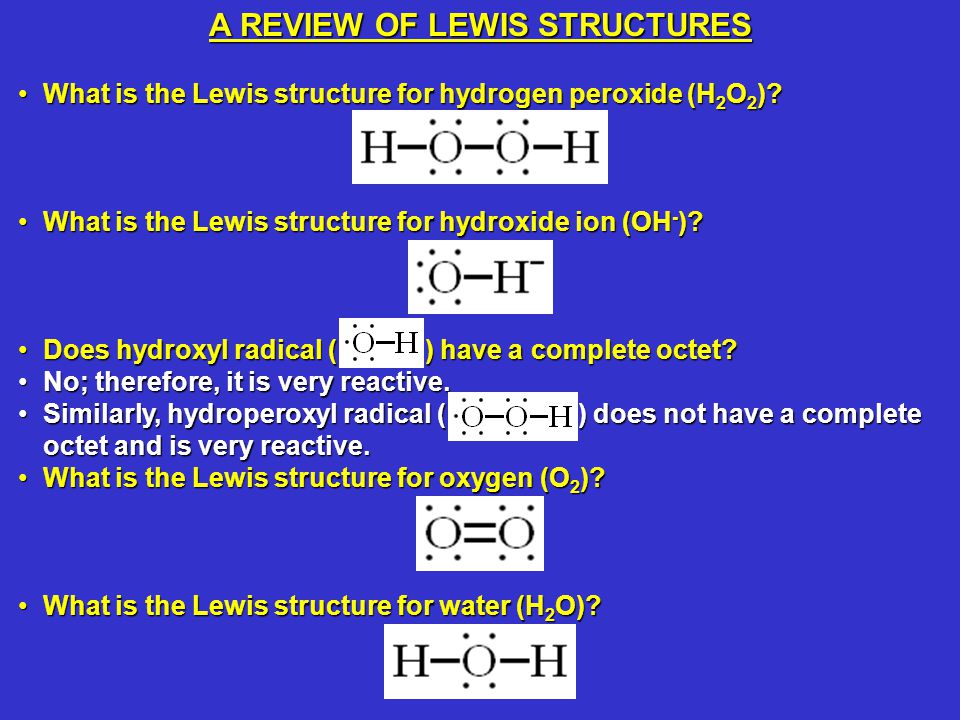


Ch 103 Percent Hydrogen Peroxide Ppt Video Online Download



Video Lewis Structure For H2o2
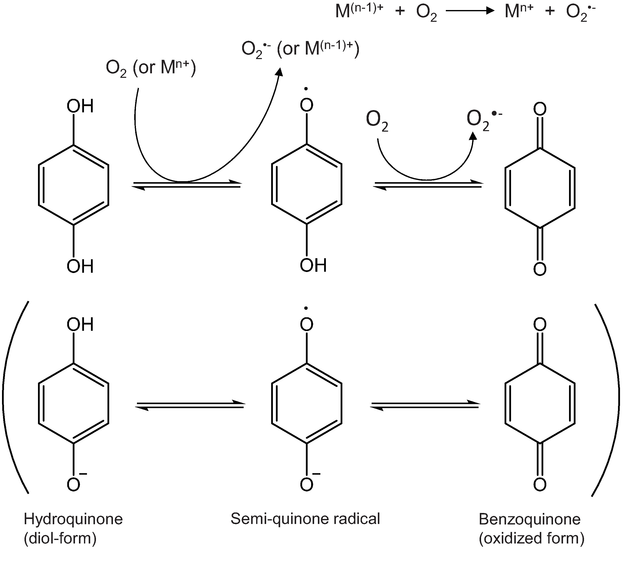


Biomolecules Oxidation By Hydrogen Peroxide And Singlet Oxygen Intechopen
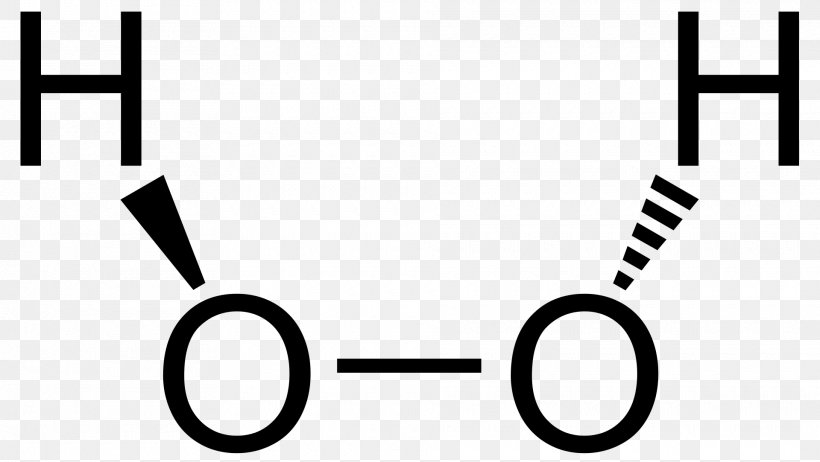


Hydrogen Peroxide Lewis Structure Chemistry Barium Peroxide Png 19x10px Hydrogen Peroxide Area Atom Barium Peroxide Black



Lewis H2o2 Janet Gray Coonce


Hydrogen Peroxide Molecule Of The Month September 06 Html Version



Ferric Mb Reacts With Hydrogen Peroxide To Give The Long Lived Download Scientific Diagram



Efficient Hydrogen Peroxide Generation Utilizing Photocatalytic Oxygen Reduction At A Triphase Interface Sciencedirect
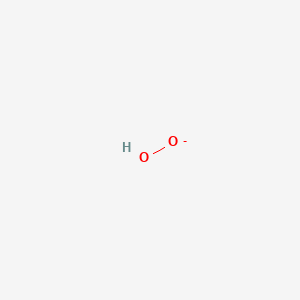


Hydrogenperoxide 1 Ho2 Pubchem



How To Make Oxygen From Permanganate And Hydrogen Peroxide Science Experiments Wonderhowto
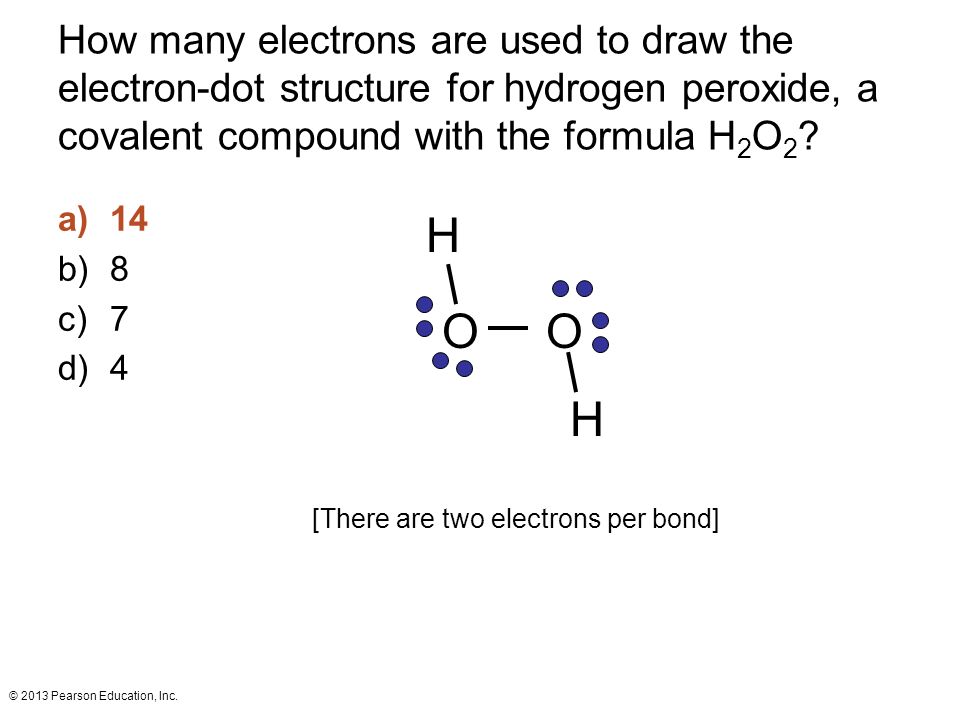


Chemical Bonds And Mixtures Ppt Video Online Download



Lewis Structure For H2o2 Learn Lif Co Id
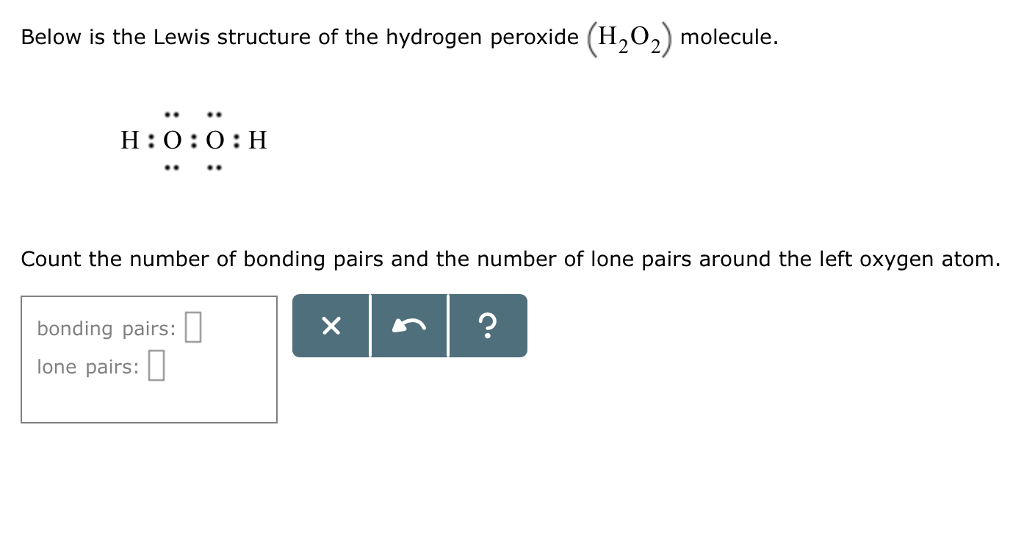


Solved Below Is The Lewis Structure Of The Hydrogen Perox Chegg Com


Rcsb Pdb 6kmk Crystal Structure Of Hydrogen Peroxide Bound Bovine Lactoperoxidase At 2 3 A Resolution



What Is The Lewis Structure For H2o2 Study Com



Reduction Of Oxygen A Single Electron Transfer Which Converts Download Scientific Diagram



Write The Lewis Structure Of Hydrogen Peroxide
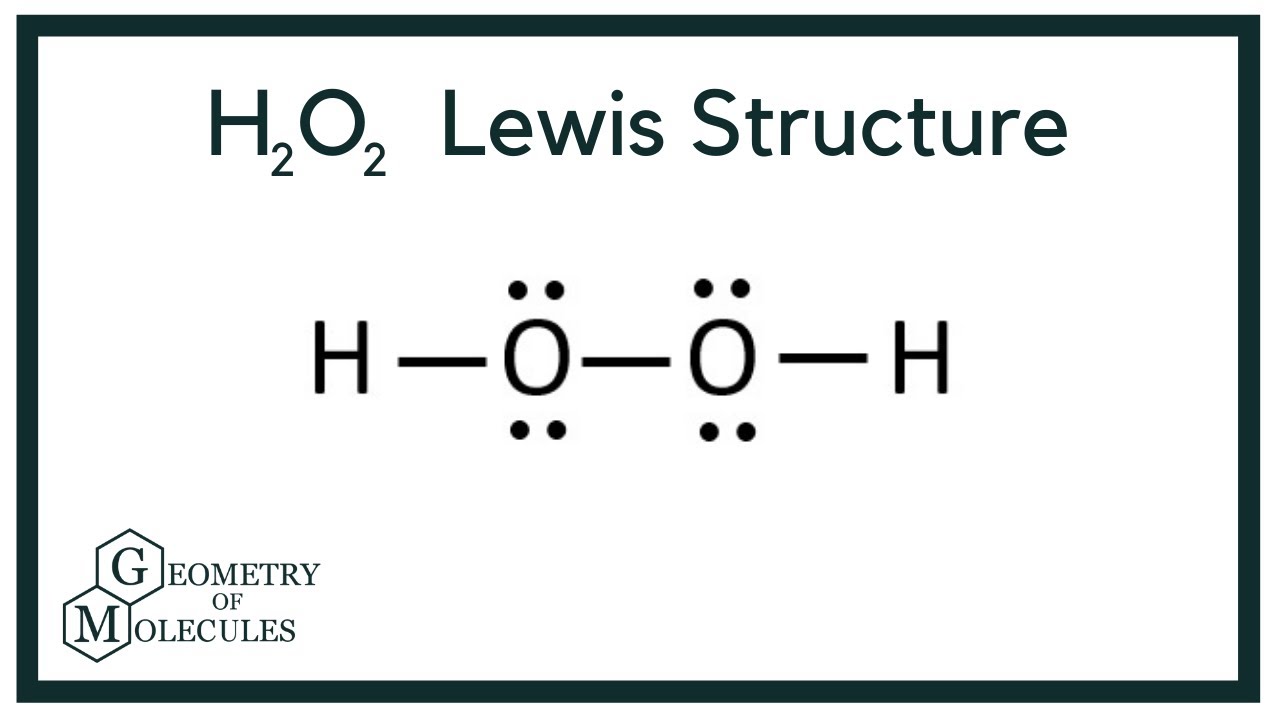


H2o2 Lewis Structure Hydrogen Peroxide Youtube



Construct A Lewis Structure For Hydrogen Peroxide H 2o 2 In Which Each Atom Achieves An Octet Of Electrons Study Com



Hydrogen Peroxide Sensing And Signaling Molecular Cell



Between The Two Structures See Image Which Is The Correct Lewis Structure For Hydrogen Peroxide Inorganic Chemistry Symmetry Of Molecules Chemistryhelp



Spontaneous Generation Of Hydrogen Peroxide From Aqueous Microdroplets Pnas
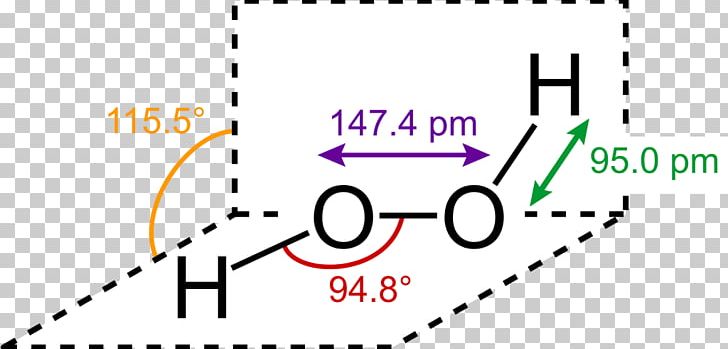


Lewis Structure Hydrogen Peroxide Molecule Structural Formula Png Clipart Angle Brand Chemical Bond Chemical Compound Chemical
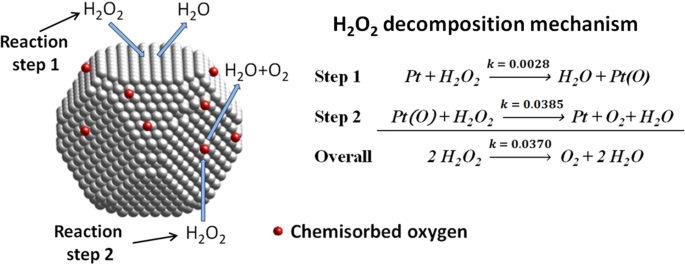


Kinetic Effect Of Surface Chemisorbed Oxygen On Platinum Catalyzed Hydrogen Peroxide Decomposition Springerlink


Lewis Structure Of H2o2


Oxygen Free Radical Electron Dot Diagram



0 件のコメント:
コメントを投稿